When a generic drug hits the market, you assume it works just like the brand-name version. But how do regulators know for sure? Traditional measures like partial AUC and Cmax used to be enough. Today, they’re often not. For complex drug formulations-especially extended-release pills, abuse-deterrent opioids, or mixed-release products-those old metrics can miss critical differences in how the drug is absorbed. That’s where partial AUC comes in.
Why Traditional Bioequivalence Metrics Fall Short
For decades, bioequivalence was judged using two numbers: Cmax (the highest concentration in the blood) and total AUC (the total drug exposure over time). If the generic’s Cmax and AUC fell within 80-125% of the brand’s, it was approved. Simple. Clean. But this approach assumes all drugs behave the same way, no matter how they’re designed. That’s not true. Take an extended-release painkiller. The brand version might release 40% of its dose in the first two hours, then slowly release the rest over 12 hours. A generic might hit the same total AUC and Cmax-but what if it releases 70% in the first two hours? The patient gets a dangerous spike in pain relief early on, then runs out of drug too soon. Total AUC doesn’t catch that. Cmax might be similar, but the shape of the curve? Totally different. This isn’t theoretical. In 2014, a study in the European Journal of Pharmaceutical Sciences found that 20% of generics that passed traditional bioequivalence tests failed when partial AUC was applied. For formulations tested under both fasting and fed conditions, failure rates jumped to 40%. The old system was letting unsafe or ineffective versions through.What Is Partial AUC (pAUC)?
Partial AUC isn’t a new kind of measurement. It’s a smarter way to use the same blood concentration data. Instead of looking at the entire curve from zero to infinity, you zoom in on a specific time window that matters clinically. For example, if a drug needs to start working within 30 minutes to control acute pain, regulators care most about exposure between 0 and 2 hours. That’s the pAUC window. Or if a drug is meant to last 12 hours, they might look at exposure between 4 and 8 hours-when the drug should be maintaining steady levels. The FDA defines pAUC in three main ways:- Based on when drug concentration exceeds a certain threshold (e.g., above 10% of Cmax)
- Based on the time to peak concentration (Tmax) of the reference product
- Based on a percentage of Cmax (like the area under the curve from 0 to 50% of Cmax)
How pAUC Changes the Game for Complex Drugs
The real power of pAUC shows up in three types of drugs:1. Extended-release formulations
A generic opioid pill might have the same total exposure as the brand, but if it releases too fast at the start, it could be abused. pAUC lets regulators check exposure in the first 1-2 hours-the window where abuse potential is highest. The FDA now requires pAUC for nearly all abuse-deterrent opioids.
2. Mixed-mode release products
Some drugs combine immediate-release and extended-release particles in one pill. Traditional AUC can’t tell if the immediate-release part is working correctly. pAUC lets you isolate and compare the early absorption phase separately.
3. Drugs with narrow therapeutic windows
Think anticoagulants, epilepsy meds, or immunosuppressants. A small difference in absorption rate can mean the difference between a seizure and a stroke. pAUC catches those subtle but dangerous shifts.
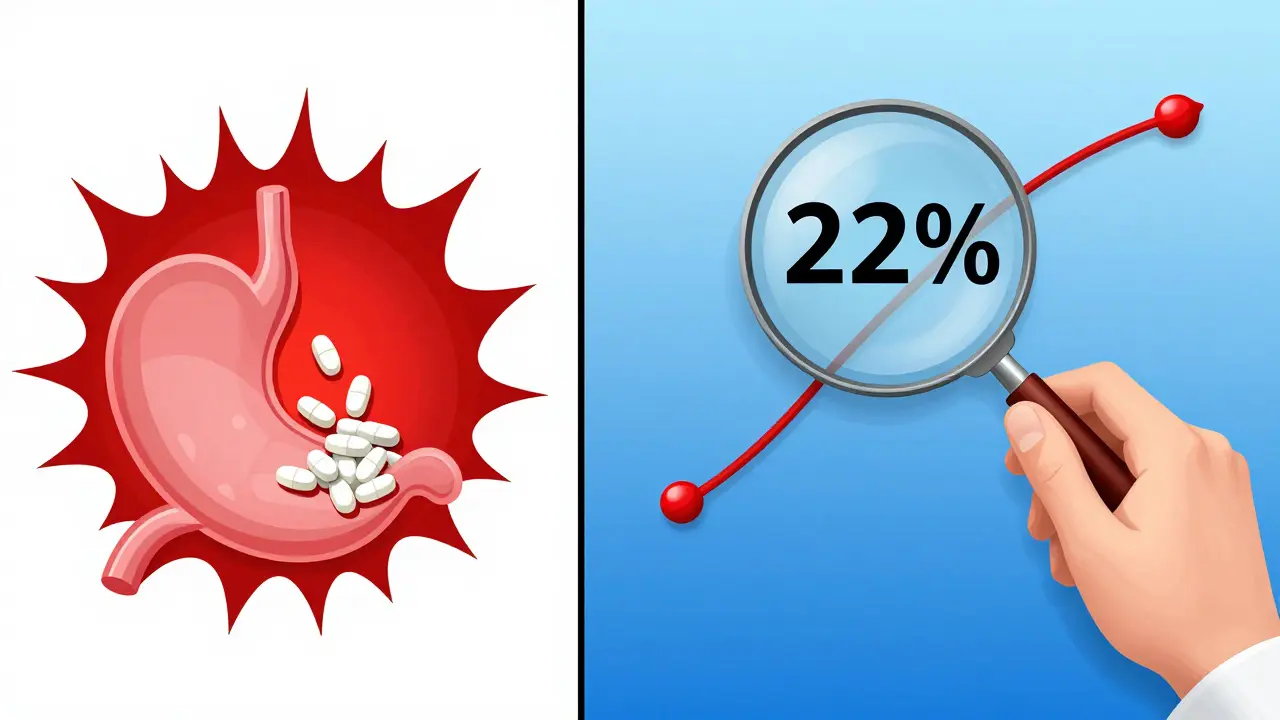
In one case presented at the 2021 AAPS meeting, a generic version of a CNS drug showed identical total AUC and Cmax to the brand. But pAUC analysis revealed a 22% lower exposure in the first 90 minutes. That difference meant the generic wouldn’t control seizures as reliably. It was pulled before it reached patients.
Regulatory Shifts: FDA and EMA Lead the Way
The push for pAUC didn’t come from academia alone. Regulators saw the gaps. The European Medicines Agency (EMA) first formally recommended pAUC in 2013 for prolonged-release products. The FDA followed, and by 2018, its Center for Drug Evaluation and Research (CDER) began standardizing its use across all drug types. Today, over 127 specific drug products have FDA-mandated pAUC requirements. That’s up from just 12 in 2021. The EMA now recommends pAUC for 27 product categories. The trend is clear: pAUC is becoming standard, not optional. The FDA’s 2023 draft guidance adds 41 more drugs to the list. By 2027, Evaluate Pharma predicts 55% of all generic approvals will require pAUC analysis. That’s up from 35% in 2022.The Hidden Costs: Why pAUC Is Hard to Implement
There’s a reason pAUC isn’t used everywhere: it’s expensive and complex.- Studies often need 25-40% more participants than traditional ones. A Teva biostatistician reported increasing sample size from 36 to 50 subjects for one opioid generic-adding $350,000 to development costs.
- Defining the right time window is tricky. If you pick the wrong interval, the whole analysis is invalid. FDA inspection reports from 2022 show 17 ANDA submissions were rejected just for incorrect pAUC time selection.
- Statistical methods are more advanced. The Bailer-Satterthwaite-Fieller method is now standard for calculating confidence intervals, but most biostatisticians weren’t trained in it.

Who’s Using pAUC-and Who’s Struggling?
Adoption isn’t equal. Large pharmaceutical companies with in-house PK/PD teams are leading the charge. 92% of pAUC implementations happen in firms with over 500 employees. Smaller companies? They’re outsourcing to specialized CROs. Algorithme Pharma, for example, now controls 18% of the complex generic bioequivalence market by offering proprietary pAUC analysis tools. Therapeutic areas with the highest pAUC use:- Central nervous system drugs: 68%
- Pain management: 62%
- Cardiovascular agents: 45%
How to Get Started with pAUC
If you’re developing a generic drug and think pAUC might be needed, here’s how to begin:- Check the FDA’s product-specific guidances. Search the FDA’s website for your drug. If pAUC is required, the guidance will say so.
- Identify the clinically relevant time window. Ask: When does the drug need to work? When does it matter most? Use the reference product’s Tmax and PD data to guide you.
- Use pilot studies. Run small-scale studies to see how your formulation behaves. Don’t guess the cutoff time-measure it.
- Partner with experts. Hire a biostatistician with pAUC experience. Most job postings for bioequivalence roles now list it as a required skill.
- Validate your method. Use software like Phoenix WinNonlin or NONMEM. Don’t rely on Excel.
The Bottom Line
Partial AUC isn’t just another statistical trick. It’s a necessary upgrade to protect patients. For simple, immediate-release drugs, traditional metrics still work fine. But for anything complex-extended-release, abuse-deterrent, or narrow-therapeutic-index drugs-pAUC is no longer optional. It’s the only way to ensure that a generic doesn’t just look similar on paper, but behaves the same in the body. The cost is higher. The process is harder. But the stakes are higher too. One missed difference in absorption rate can mean a patient doesn’t get relief-or worse, has a dangerous reaction. The science is solid. The regulators are pushing. The industry is adapting. If you’re in generic drug development, ignoring pAUC isn’t cutting corners. It’s gambling with patient safety.Is partial AUC required for all generic drugs?
No, partial AUC is only required for specific drug products with complex release profiles-like extended-release, abuse-deterrent, or mixed-mode formulations. For simple immediate-release drugs, traditional Cmax and total AUC are still sufficient. Always check the FDA’s product-specific guidance for your drug.
How is pAUC different from total AUC?
Total AUC measures total drug exposure over the entire time course, from dosing to when the drug is fully cleared. Partial AUC measures exposure only during a specific, clinically relevant time window-like the first 2 hours after dosing. This lets regulators focus on absorption patterns that matter for safety and effectiveness, not just overall exposure.
What time window should I use for pAUC?
There’s no universal rule. The time window must be tied to a clinically relevant pharmacodynamic effect. For example, if a drug needs to work within 30 minutes, use 0-2 hours. For drugs meant to last 12 hours, you might look at 4-8 hours. Use the reference product’s Tmax and published PD data to define the window. The FDA recommends aligning it with the time when the drug’s effect is most sensitive to concentration changes.
Why do pAUC studies need more participants?
pAUC focuses on a smaller portion of the concentration-time curve, which often has higher variability between subjects. Because you’re measuring a narrower window, small fluctuations in absorption have a bigger impact on the result. To maintain statistical power, you need more people. Studies show sample sizes often need to increase by 25-40% compared to traditional bioequivalence studies.
Can I use Excel to calculate pAUC?
Technically, yes-but you shouldn’t. Excel lacks the precision and validation needed for regulatory submissions. The FDA requires use of validated software like Phoenix WinNonlin, NONMEM, or PKanalix. These tools properly handle nonlinear interpolation, log-transformation, and statistical modeling required for pAUC analysis. Using Excel increases the risk of rejection during FDA review.
Is pAUC used outside the U.S. and Europe?
Currently, the FDA and EMA are the main drivers of pAUC adoption. Other agencies, like Health Canada and PMDA in Japan, are watching closely but haven’t mandated it broadly yet. The International Consortium for Innovation and Quality in Pharmaceutical Development (IQ Consortium) reports that inconsistent global standards add 12-18 months to global generic drug development timelines. Expect wider adoption as more countries align with FDA and EMA guidelines.







13 Comments
Finally someone broke this down without the jargon overload. I work in pharmacy and this is exactly why I hate when generics get approved without checking the absorption curve. One pill looks the same on paper, but if it spikes too fast, patients get nauseous or worse.
Let me be clear: if your generic doesn’t pass pAUC, it’s not bioequivalent-it’s a liability waiting for a lawsuit. The FDA isn’t being pedantic. They’re preventing deaths. And if you’re still using Excel to calculate AUC, you’re not a scientist-you’re a liability.
Y’all are overcomplicating this. If it looks the same and costs less, why does it matter? People just want pain relief. Stop making drugs so damn complicated.
From India, we’re watching this closely. Our local manufacturers are struggling with the cost and complexity. But I agree-better safe than sorry. A patient dying because of a bad generic? That’s not a business problem. That’s a moral failure. We need more training, not more excuses.
There’s a deeper philosophical question here: if we can’t measure what matters, do we truly understand the drug? Traditional metrics are convenient, but convenience is not truth. pAUC forces us to confront the asymmetry between mathematical equivalence and physiological reality.
Let’s not pretend this is about patient safety-it’s about regulatory capture. The big pharma conglomerates pushed pAUC to create barriers to entry. Smaller firms can’t afford the 50-subject trials, so they get squeezed out. It’s not science-it’s monopoly maintenance disguised as clinical rigor.
Interesting read. I’m not in pharma, but I’ve had to take a few of these extended-release meds. I never realized how much timing mattered. If the generic hits too fast, I get the buzz, then crash an hour later. Feels like the drug is lying to me.
The statistical methods underpinning pAUC are not just advanced-they’re *nonlinear*. The Bailer-Satterthwaite-Fieller approach accounts for heteroscedasticity in the early absorption phase, which traditional ANOVA completely ignores. Most bioequivalence analysts still default to log-transformed Cmax/AUC because they’re trained on 1990s protocols. We’re decades behind in methodological literacy.
Just to clarify something: pAUC isn’t replacing total AUC-it’s augmenting it. Think of it like adding a high-res camera to a blurry photo. You still need the whole picture, but now you can see the fine details that matter. If you’re developing a generic, don’t treat pAUC as extra work. Treat it as your safety net.
I’ve seen this play out in the UK. One brand switched to abuse-deterrent formulation, and the generics kept getting pulled. The regulators were right. People were crushing them to get high. pAUC caught the early spike. It’s not perfect, but it’s a step toward real accountability.
The imposition of pAUC as a regulatory standard represents an epistemological shift from reductionist pharmacokinetics toward phenomenological pharmacodynamics. The body is not a beaker. The curve is not a line. To reduce therapeutic equivalence to a percentage interval is to commit a category error of the highest order.
Wait-so the FDA is making us test for absorption timing because Big Pharma doesn’t want generics to work? I heard this is all about patent extensions. They’re using ‘patient safety’ as a cover to keep prices high. I’ve got a cousin who got seizures because of a generic-turned out the brand changed their formulation and the generics didn’t catch up. Coincidence? I think not.
Shawn, you’re right that it’s complicated. But you’re also ignoring that people die because someone cut corners. I’ve reviewed ANDA submissions. The ones that skip pAUC? They’re the ones that come back with ‘serious safety concerns’ six months later. You want cheap? Fine. But don’t pretend you’re saving money when you’re just shifting risk to the patient.