
When a pharmacist swaps a brand-name drug for a generic version, it’s not just a simple switch. Behind that decision is a legal, clinical, and administrative process that must be recorded properly - or it can lead to errors, insurance denials, or even patient harm. In 2025, with over 90% of prescriptions filled with generics in the U.S., generic substitution is routine. But documenting it right? That’s where things get complicated.
Why Documentation Matters More Than You Think
Generic drugs are chemically identical to their brand-name counterparts. They contain the same active ingredient, dose, and route of administration. The FDA requires them to be bioequivalent - meaning they deliver the same amount of drug into the bloodstream within a narrow range (80-125% of the brand). That’s the science. But biology isn’t always predictable. A patient on warfarin, for example, might be stable on one generic manufacturer’s version. Switch to another - even if it’s labeled "therapeutically equivalent" - and their INR levels can spike or drop. One study in the Journal of the American Pharmacists Association showed that poor documentation of a substitution led to a hospitalization because the prescriber didn’t know the brand had changed. That’s why documentation isn’t just paperwork. It’s a safety net.What Exactly Must Be Documented?
There’s no single federal rule. Every state has its own requirements. But most follow a core set of data points that must be recorded at the time of dispensing:- The brand name prescribed by the doctor
- The generic name of the drug dispensed
- The manufacturer or distributor name
- The lot number
- The expiration date
State Laws Are a Patchwork - And That’s a Problem
There are 50 states. Each has different rules on who can authorize a substitution and whether patient consent is needed.- 27 states let pharmacists substitute without asking the patient.
- 14 states require explicit, documented patient consent.
- 9 states use a mix - consent for NTI drugs, automatic for others.
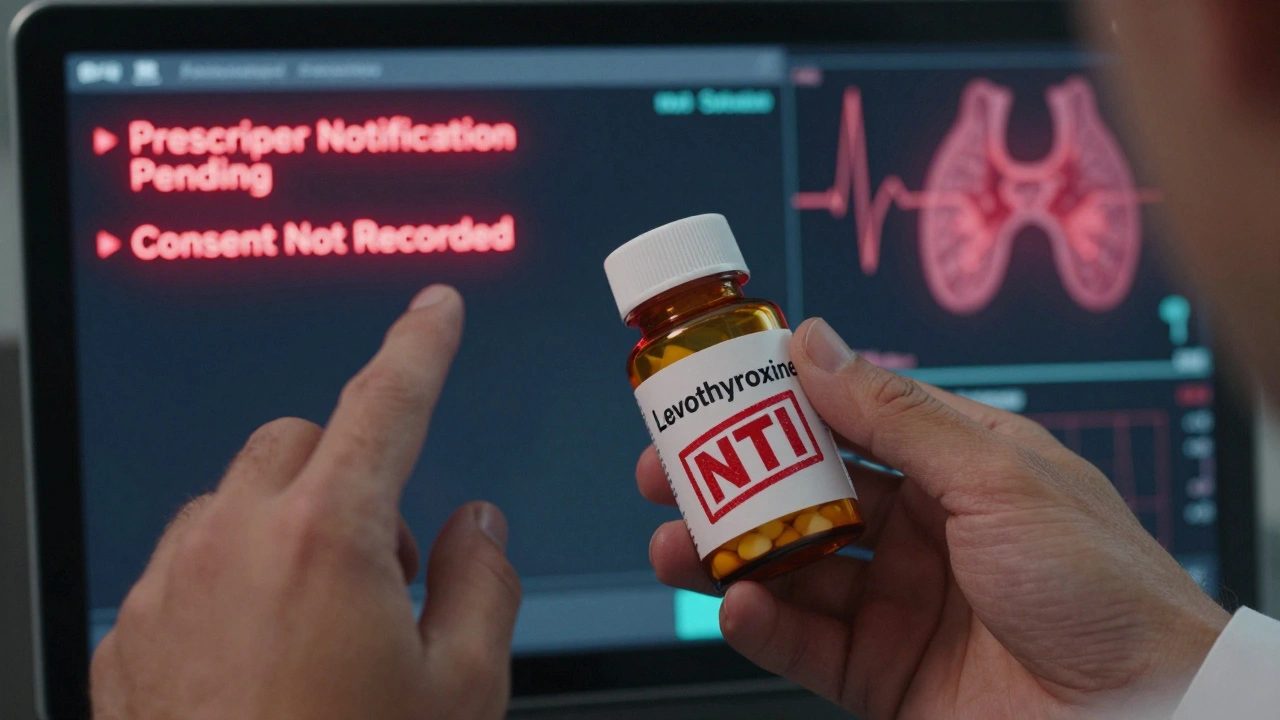
Electronic Systems Are the New Normal - But They’re Not Perfect
Most pharmacies use electronic health records (EHRs) or pharmacy management systems like Epic, Cerner, or QS/1. But here’s the catch: 32% of pharmacies using Epic reported needing custom configurations just to meet state documentation rules. That’s not a bug - it’s a feature gap. The system might auto-populate the generic name and manufacturer. But does it prompt the pharmacist to enter the lot number? Does it flag NTI drugs? Does it send a notification to the prescriber if substitution occurs? If not, the pharmacist has to manually add it - and that’s where mistakes happen. California’s SB 564, effective January 1, 2024, changed the game. It now requires real-time electronic documentation of substitutions that prescribers can see instantly through their EHR. Other states are watching. If this becomes standard, pharmacists won’t just be documenting - they’ll be sharing critical data in real time.Who’s Responsible for What?
The pharmacist is the one who dispenses. So they’re the one who documents. But it’s not a solo act. - Prescribers must document if they write "Do Not Substitute" or "Brand Necessary" on the prescription. The American Medical Association recommends this for all chronic conditions, especially mental health or cardiac drugs. - Patients have the right to refuse a substitution. That refusal must be documented - even if it’s just a checkbox in the system. - Regulators (state boards of pharmacy) audit records. If they find a missing lot number or no consent on file, fines can start at $1,000 per violation. The World Medical Association says: once a patient is stable on a drug - brand or generic - don’t switch without the prescriber’s approval. That’s especially true for long-term medications. Documentation here isn’t about compliance. It’s about continuity of care.Common Mistakes and How to Avoid Them
Pharmacists aren’t trying to cut corners. But the pressure to move fast - and the complexity of rules - leads to errors:- Missing lot numbers - Most systems auto-fill, but if the system crashes or the barcode doesn’t scan, the pharmacist forgets to enter it manually.
- Confusing generic names - Two different generics for the same drug might have different inactive ingredients. If the patient is allergic to a dye in one, but not the other, that matters.
- Delaying documentation - 41 states require records to be completed within 24 hours. Waiting until the end of the shift? That’s a violation.
- Assuming all generics are equal - The Orange Book says two generics are therapeutically equivalent. But if a patient had a reaction to one, the next one might be different. Document the reaction - not just the substitution.

What’s Changing in 2025?
The landscape is shifting fast: - The FDA’s GDUFA III rules (2022) now require manufacturers to submit more detailed bioequivalence data for complex generics - like inhalers or injectables. That means more scrutiny on what’s being substituted. - The Medicare Part D program now demands clear substitution notes for audit purposes. If you can’t prove you documented a generic swap, the pharmacy won’t get paid. - A pilot using blockchain to track substitutions (National Pharmaceutical Council, 2023) cut documentation errors by 22%. It’s not mainstream yet - but it’s coming. - The Department of Health and Human Services is drafting national guidelines for substitution documentation. A draft is expected in mid-2024. If adopted, it could end the 50-state patchwork. For now, pharmacists must stay updated. The National Community Pharmacists Association offers a free, quarterly updated tool that cross-references state laws with documentation rules. Bookmark it. Print it. Use it.Final Takeaway: Documentation Is Clinical Care
You’re not just filling a prescription. You’re managing risk. Every time you swap a brand for a generic, you’re changing a patient’s treatment. The documentation you leave behind is the only thing that tells the next pharmacist, the prescriber, or the emergency room doctor what happened. It’s not about checking boxes. It’s about making sure no one falls through the cracks.Do I need patient consent to substitute a generic drug?
It depends on your state. In 27 states, pharmacists can substitute without asking. In 14 states, you must get explicit, documented consent. Nine states use a hybrid approach - requiring consent for certain drugs like NTI medications. Always check your state’s pharmacy board rules before substituting.
What happens if I forget to document a generic substitution?
Failure to document can lead to insurance claim denials, pharmacy audits, fines, or even license suspension. Some states impose fines of $500-$5,000 per violation. More importantly, incomplete records can lead to medication errors - like a patient receiving the wrong generic version for a critical drug, resulting in hospitalization.
Are all generic drugs truly interchangeable?
FDA says yes - if they’re rated as therapeutically equivalent in the Orange Book. But real-world experience shows exceptions. For drugs with a narrow therapeutic index - like warfarin or levothyroxine - even small differences in inactive ingredients can affect absorption. Always document any adverse effects after a substitution, even if the patient says they’re fine.
Can a prescriber prevent generic substitution?
Yes. Prescribers can write "Do Not Substitute" or "Brand Necessary" on the prescription. In many states, this overrides the pharmacist’s ability to substitute - even if a generic is available. Pharmacists must honor these instructions. Documenting this prevents legal liability and ensures patient safety.
How often do I need to update my knowledge of state substitution laws?
At least quarterly. State laws change often - especially with new legislation like California’s SB 564 in 2024. Use trusted resources like the National Community Pharmacists Association’s online tool, which updates every three months. Don’t rely on memory or outdated handbooks.
Is electronic documentation required?
Not federally, but 98% of chain pharmacies and 87% of independents now use electronic systems for substitution records. Many states now require it - especially for real-time reporting to prescribers. Paper logs are becoming obsolete and are harder to audit. If your system doesn’t auto-populate substitution fields, request an upgrade.
Next Steps for Pharmacists
If you’re new to this, start here:- Go to your state board of pharmacy website and download the current substitution law.
- Check your pharmacy software: Does it auto-fill all required fields? Can it flag NTI drugs?
- Train your team on the 5 key data points - lot number, manufacturer, brand, generic, expiration.
- Set a daily reminder to review 5 random substitution records for completeness.
- Bookmark the NCPA substitution tool - update it every quarter.







11 Comments
So let me get this straight - we’re now treating pharmacists like human barcode scanners with a side of legal liability? 🤯 I just got my levothyroxine swapped and the pharmacist didn’t even blink. Now I’m supposed to trust that some random lot number from a factory in Bangalore is gonna keep my thyroid from staging a coup? I’m not mad… I’m just disappointed in capitalism.
Let’s be real - this is why America’s healthcare system is broken. We’ve got 50 different state laws for documenting a pill swap? That’s not compliance, that’s bureaucratic chaos. Meanwhile, China and Germany have national standards. We’re still fighting over whether to require consent for generic lisinopril. Fix the system, not the paperwork.
Documentation = modern alchemy. Turning a simple Rx into a blockchain-ready sacrament. 🕊️💊 We’re not just filling prescriptions - we’re curating patient narratives for the gods of HIPAA. The lot number? It’s the soul of the pill. Don’t you dare skip it.
It’s fascinating how we’ve anthropomorphized pharmaceutical regulation - as if the FDA’s Orange Book is some sacred scripture. But let’s not pretend bioequivalence is a binary truth. The pharmacokinetic variance between generics? It’s not noise - it’s a symphony of micro-differences. And we’re pretending they’re interchangeable because it’s cheaper. That’s not science. That’s economic nihilism dressed in white coats.
They’re lying. They’re ALL lying. The FDA doesn’t test generics properly - the manufacturers self-report. The lot numbers? Fake. The ‘therapeutic equivalence’? A corporate marketing ploy. And now they want us to trust electronic logs? HA. Your EHR is hacked. Your pharmacist’s system is running on Windows XP. This isn’t safety - it’s a surveillance trap. #PharmaCoverup
Bro… in India we don’t even have generic labeling rules. Some pills come in packets with no manufacturer name - just a scribble and a prayer. And you’re stressing over lot numbers? I’ve seen people take 3 different generics for the same drug because the shopkeeper said ‘same thing’. No documentation. No audits. Just vibes. Maybe we should export this chaos to the US? 😅
Let’s pause. This isn’t just about paperwork - it’s about dignity. Every time a pharmacist documents a substitution, they’re saying: ‘I see you. Your body matters. Your history matters.’ This isn’t bureaucracy - it’s care in motion. We treat patients like data points, but every lot number? That’s a human being’s stability. Don’t reduce this to compliance. Reduce it to compassion.
Just had my 3rd generic switch this year. Felt fine. But now I’m paranoid. Should I have asked for the brand? Do I need to keep a journal? Is my thyroid judging me? Anyway - love that we’re finally talking about this. My pharmacist actually asked if I wanted the brand this time. Small win. Keep pushing for better systems - we got this.
I’m a pharmacist in rural Oregon. We don’t have fancy EHRs - we use paper logs and a sticky note system. But I write EVERYTHING. Lot number? Check. Manufacturer? Check. Patient said they felt ‘weird’ after the last swap? I wrote it down. One time, a patient came back with a rash - turned out the dye in the generic was the issue. We caught it because I documented. This isn’t about rules - it’s about showing up. For real.
Wow. So now pharmacists are expected to be clinical historians, legal analysts, and data entry clerks - all while being yelled at by patients who think ‘generic’ means ‘cheap junk’. And the system rewards them with… what? A 3% raise? This isn’t healthcare. It’s a performance art piece called ‘The Burden of the White Coat’.
It is with profound reverence for the sanctity of patient care that I offer this reflection: the meticulous documentation of generic substitution is not merely an administrative obligation, nor is it a bureaucratic impediment - it is, in fact, a sacred covenant between the practitioner and the patient, a tangible manifestation of the Hippocratic Oath in the digital age. Each lot number, each manufacturer designation, each consent form - these are not data points; they are echoes of trust, reverberating through time, safeguarding the fragile equilibrium of human physiology against the tide of commodification. Let us not underestimate the weight of the pen - or the keystroke - for in its movement lies the preservation of life itself.